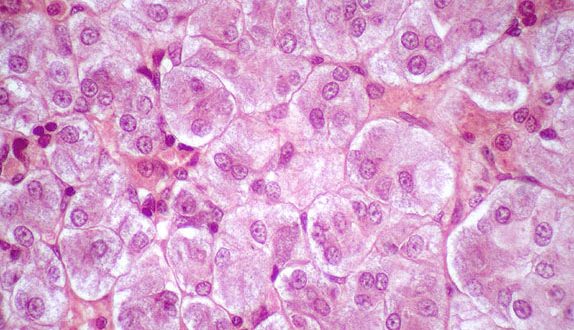
Researchers have found a new and effective method to acquire viable cells from cryopreserved tissue samples, which could help researchers to collect and analyse samples from different study sites to conduct more centralized research.
Rheumatoid arthritis (RA) is a chronic disease that causes pain, stiffness, swelling, and limitation in the motion and function of multiple joints. Though joints are the principal body parts affected by RA, inflammation can develop in other organs as well. An estimated 1.3 million Americans have RA, and the disease typically affects women twice as often as men.
The Accelerating Medicines Partnership (AMP) program’s RA/SLE Network aims to discover new, more effective ways to treat the inflammatory response that causes these chronic diseases by identifying the cell types and signals central to driving inflammation in RA patients. Before the program’s researchers could conduct the analysis required to identify new therapeutic strategies, they first needed a standardized method to gather viable cells from tissues samples gathered at different study sites in the network.
“Performing studies like these on a large scale requires the participation of many different institutions. A major challenge the investigators faced is how to analyze samples collected at different sites across the United States and United Kingdom in a uniform, reproducible way and still be able to use the specialized technologies that are only available at a few research institutions,” said Deepak Rao, MD, PhD, Co-Director of the Human Immunology Center at Brigham and Women’s Hospital in Boston, and a lead author of the study.
The researchers devised a method to freeze intact synovial tissue samples soon after acquisition so that they could be stored, transported and then analyzed at a single technology site. The pipeline was designed to generate three detailed datasets on each synovial sample in a uniform way such that all of the samples could be compared.
The cryopreserved tissue dissociated by these new methods could be analyzed by multiple, high-dimensional analyses. Mass cytometry revealed diverse fibroblast phenotypes, clear separation of memory B cells from antibody-secreting cells, and multiple phenotypes of activated CD4+ and CD8+ T cells. To complement the mass cytometry analysis, the researchers developed a flow cytometric sorting strategy to collect fibroblasts, macrophages, T cells and B cells for bulk and single cell RNA-sequencing transcriptomics.
The researchers believe this new method to acquire viable cells from cryopreserved synovial tissue provides a powerful method to analyze joints samples from a large number of RA patients using multiple, robust, high-dimensional analyses.
“When applied to large numbers of patients, these analyses have the potential to identify specific inflammatory pathways that define subsets of RA patients, which may eventually help guide treatment decisions,” said Dr. Rao. “We also hope that this approach of cryopreserving viable, intact tissue can be used as a model for centralized analysis of samples from multiple study sites in other rheumatic and inflammatory conditions.”
Agencies/Canadajournal
 Canada Journal – News of the World Articles and videos to bring you the biggest Canadian news stories from across the country every day
Canada Journal – News of the World Articles and videos to bring you the biggest Canadian news stories from across the country every day