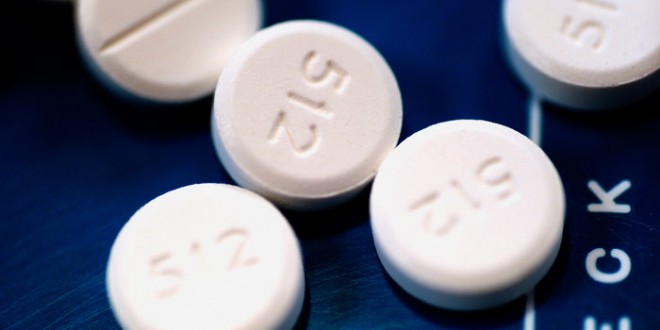
The Food and Drug Administration said Tuesday that all fast-acting opioid pain medicines will be required to carry its strongest warning about risks, including the risks for abuse, addiction, overdose and death.
Labeling changes will add more warnings to prescription opioids like oxycodone, hydrocodone and morphine. A warning with the drugs’ packaging will advise people that the medications have risks including addiction, misuse, overdose and death. In a press conference about the change, newly minted FDA commissioner Dr. Robert Califf said the new requirements are part of several steps the FDA is taking to “reverse this epidemic.” The FDA says it’s “reassess[ing] its approach to opioid medications” in general.
The agency’s goal is to help curb the opioid epidemic in the U.S. while making sure people are still getting pain relief. The warnings emphasize that prescription painkillers should be used only when necessary, and as a last resort. New warnings also include the risk of women using opioids while pregnant, which has been linked to opioid withdrawal syndrome (NOWS) in newborns.
The U.S. Centers for Disease Control and Prevention (CDC) also recently released guidelines for opioid use, arguing physicians should be conservative when prescribing the drugs. The group also recommended doctors offer alternatives, like exercise and cognitive behavioral therapy or prescribing the lowest possible doses.
Agencies/Canadajournal
 Canada Journal – News of the World Articles and videos to bring you the biggest Canadian news stories from across the country every day
Canada Journal – News of the World Articles and videos to bring you the biggest Canadian news stories from across the country every day