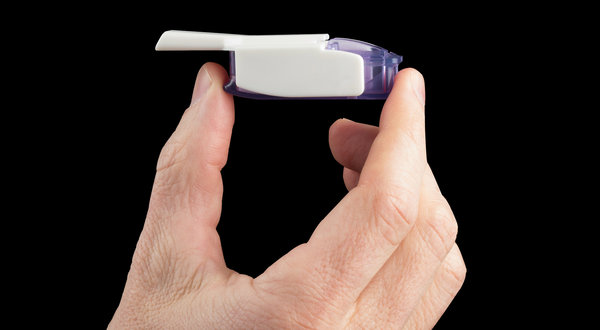
An FDA advisory panel has voted in favor of approving a new inhaled insulin device, Afrezza, as a short-acting treatment to improve glycemic control in both type 1 and type 2 diabetes.
The Endocrinologic and Metabolic Drugs Advisory Committee voted 13-1 here Tuesday to support approval of the device to treat adult patients with type 1 diabetes, agreeing that the device manufacturer, MannKind, demonstrated adequate evidence of safety and efficacy. One panelist who left early did not vote.
“We are pleased with the Advisory Committee’s approval recommendation in support of Afrezza, and we appreciate the thoroughness of their review,” said Alfred Mann, chairman and chief executive officer of MannKind Corporation. “We look forward to working with the FDA as they complete their evaluation of Afrezza. Diabetes is a major health problem in the United States, and we are committed to bring Afrezza to the many patients who might benefit from this novel product.”
The FDA is not bound by the Advisory Committee’s recommendation but will consider its guidance in reviewing the New Drug Application (NDA) that was submitted for Afrezza. The Prescription Drug User Fee Act (PDUFA) date for the FDA to complete its review of Afrezza is April 15, 2014.
Agencies/Canadajournal
 Canada Journal – News of the World Articles and videos to bring you the biggest Canadian news stories from across the country every day
Canada Journal – News of the World Articles and videos to bring you the biggest Canadian news stories from across the country every day