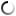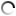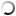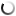U of T Scientists have discovered the literal “missing link” needed to produce a homegrown human in the lab; a finding they claim could eventually lead to the repair or replacement of damaged organs.
Led by Milica Radisic, a professor in the Institute of Biomaterials and Biomedical Engineering and the department of chemical engineering, and including graduate student Boyang Zhang, the team has found a way to manufacture small, intricate scaffolds for individual cells to grow on. These artificial environments produce cells and tissues that resemble real human cells and tissues more closely than those grown lying flat in a petri dish.
The team’s recent innovations include Biowire™, a method of growing heart cells around a silk suture, as well as a scaffold for heart cells that snaps together like sheets of Velcro™.
AngioChip takes tissue engineering to a new level. “It’s a fully three-dimensional structure complete with internal blood vessels,” says Radisic. “It behaves just like vasculature, and around it there is a lattice for other cells to attach and grow.” The technology is described in the journal Nature Materials.
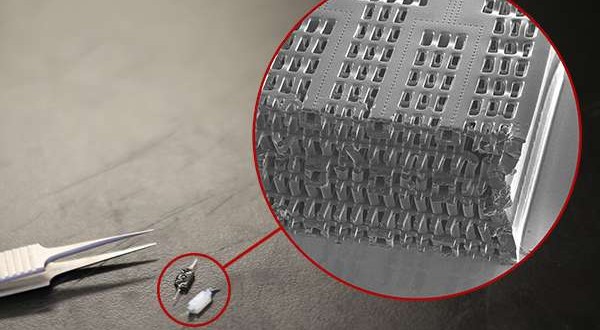
Zhang built the scaffold out of POMaC, a polymer that is both biodegradable and biocompatible. The scaffold comprises a series of thin layers, each stamped with a pattern of channels that are 50 to 100 micrometres wide (shown at left: image by Tyler Irving/Boyang Zhang/Kevin Soobrian). The layers, which resemble computer microchips, are then stacked into a 3D structure of synthetic blood vessels. As each layer is added, UV light is used to cross-link the polymer and bond it to the layer below.
When the structure is finished, it is bathed in a liquid containing living cells. The cells quickly attach to the inside and outside of the channels and begin growing as they would in the human body.
“Previously, people could only do this using devices that squish the cells between sheets of silicone and glass,” says Radisic. “You needed several pumps and vacuum lines to run just one chip.
“Our system runs in a normal cell culture dish, and there are no pumps. We use pressure heads to perfuse media through the vasculature. The wells are open, so you can easily access the tissue.”
Using the platform, the team has built model versions of both heart and liver tissues that function like the real thing. “Our liver actually produced urea and metabolized drugs,” Radisic says.
The platform can connect the blood vessels of the two artificial organs, thereby modelling not just the organs themselves but their interactions. The researchers have even injected white blood cells into the vessels and watched as they squeezed through gaps in the vessel wall to reach the tissue on the other side, just as they do in the human body.
AngioChip has great potential in the field of pharmaceutical testing. Current drug testing methods, such as animal testing and controlled clinical trials, are costly and fraught with ethical concerns. Testing on lab-grown human tissues would provide a realistic model at a fraction of the cost, but this area of research is still in its infancy.
“In the last few years, it has become possible to order cultures of human cells for testing, but they’re grown on a plate, a two-dimensional environment,” Radisic says. “They don’t capture all the functional hallmarks of a real heart muscle, for example.”
A more sophisticated platform like AngioChip could enable drug companies to detect dangerous side effects and interactions between organ compartments long before their products reach the market, saving countless lives. It could also be used to understand and validate the effectiveness of current drugs and even to screen libraries of chemical compounds to discover new drugs. The team is already working on commercializing the technology through TARA Biosystems Inc., a spin-off company co-founded by Radisic.
Radisic envisions her lab-grown tissues being implanted to repair organs damaged by disease. Because the cells used to seed the platform can come from anyone, the new tissues could be genetically identical to the intended host, reducing the risk of organ rejection. Even in its present form, AngioChip can be implanted into a living animal, its artificial blood vessels connected to a real circulatory system. The polymer scaffolding itself simply biodegrades after several months.
The team still has work to do. Each AngioChip is made by hand. If the platform is to be used industrially, the team will need to develop high-throughput manufacturing methods to create many copies at once.
Still, the potential is obvious. “It really is multifunctional, and solves many problems in the tissue engineering space,” says Radisic. “It’s truly next-generation.”
Agencies/Canadajournal
 Canada Journal – News of the World Articles and videos to bring you the biggest Canadian news stories from across the country every day
Canada Journal – News of the World Articles and videos to bring you the biggest Canadian news stories from across the country every day