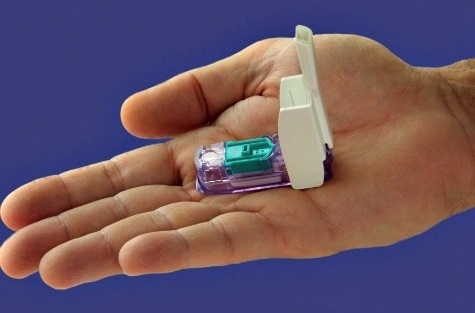
The Food and Drug Administration has approved an inhalable form of insulin to help adults with diabetes control their blood sugar while eating.
On Friday, the FDA approved Afrezza, a fast-acting powdered form of insulin that comes in small single-use cartridges.
The drug maker says the powder should be inhaled within 20 minutes of the start of a meal, and can take as little as 12 to 15 minutes to go into effect. Injectable insulin takes about half an hour.
Approval was based on studies of about 3,000 patients with type 1 or type 2 diabetes. Hypoglycemia, cough, and throat pain or irritation were the most common adverse effects associated with treatment.
At a meeting in April, an FDA advisory panel voted 13-1 that the data supported approval for treatment of adults with type 1 diabetes, and 14-0 that the data supported approval for treatment of adults with type 2 diabetes, although they had some concerns about the potential for lung cancer, acute bronchospasm, and other safety issues.
Panelists also noted that trials indicated that the inhaled insulin was not as effective as traditional insulin, and that it would not be appropriate for all patients with type 1 and type 2 diabetes, but agreed that more treatment options were needed.
The label includes a boxed warning about acute bronchospasm, which has been reported in patients with asthma and chronic obstructive pulmonary disease who have used Afrezza; it is contraindicated in patients with chronic lung disease.
To address the risks of bronchospasm, approval includes a Risk Evaluation and Mitigation Strategy (REMS), which will inform health care professionals about the risk of serious acute bronchospasm associated with treatment. The FDA is also requesting that the manufacturer, MannKind Corporation, conduct several postmarketing studies, including a study to evaluate the product in pediatric patients and a study to evaluate the potential risk of pulmonary malignancies, cardiovascular risk, and long-term pulmonary function effects.
Agencies/Canadajournal
 Canada Journal – News of the World Articles and videos to bring you the biggest Canadian news stories from across the country every day
Canada Journal – News of the World Articles and videos to bring you the biggest Canadian news stories from across the country every day